Copper dressings, as opposed to silver dressings, enhance wound healing
Copper, in addition to being a potent biocide (1), is one of the 11 essential trace minerals needed by human body as it is involved in many physiological processes in all human tissues (2). Copper is indispensable for many wound healing-related processes (3;4). It stimulates a) angiogenesis (5) by upregulating Hypoxia Induced Factor 1alpha (HIF-1α) (6), vascular endothelial growth factor (VEGF) (7), and Cu-dependent transcription factor Atox1 (8); b) expression of integrin (9); c) stimulation of secretion of fibrinogen, elastin and collagen by dermal fibroblasts (10;11) and their stabilization (12;13); d) upregulation of copper-dependent enzymes and polysaccharides, such as lysyl oxidase, metalloproteinases, glycosaminoglycans and small proteoglycans, important for matrix remodeling, cell proliferation and re-epithelization (14-17) and e) migration of skin and stem cells (18;19).
MedCu wound dressing, which are based on Cupron’s platform technology under licence, in addition of having potent antimicrobial properties as described below and in (20), serve as a reservoir of copper ions that are slowly liberated into the wounds, where they readily stimulate wound healing. This was demonstrated in diabetic mice (6) and the substantiated mechanism of enhanced wound healing by the dressings was described (6). Already in 2010, we demonstrated that the upregulation of HIF-1α played a central role in the mechanism of the observed enhanced wound healing by the copper wound dressings. Interestingly, the scientist who discovered HIF-1α received the Nobel Prize for Medicine in 2019.
In contrast, silver, which is not metabolized by the human body, has been found to downregulate HIF-1α (21), explaining at least in part the several reports of toxicity of silver wound dressings, especially to keratinocytes and fibroblasts (22). As such, in a comprehensive review conducted on the effect of silver dressings on wound healing, it was concluded that there is some evidence, although poor, that short-term use silver dressings may be helpful in infected wounds, silver dressings are not appropriate for non-infected wounds and surgical incisions (22).
The different effects of copper and silver on the very important wound healing related factor HIF-1α are depicted in Figure 1.
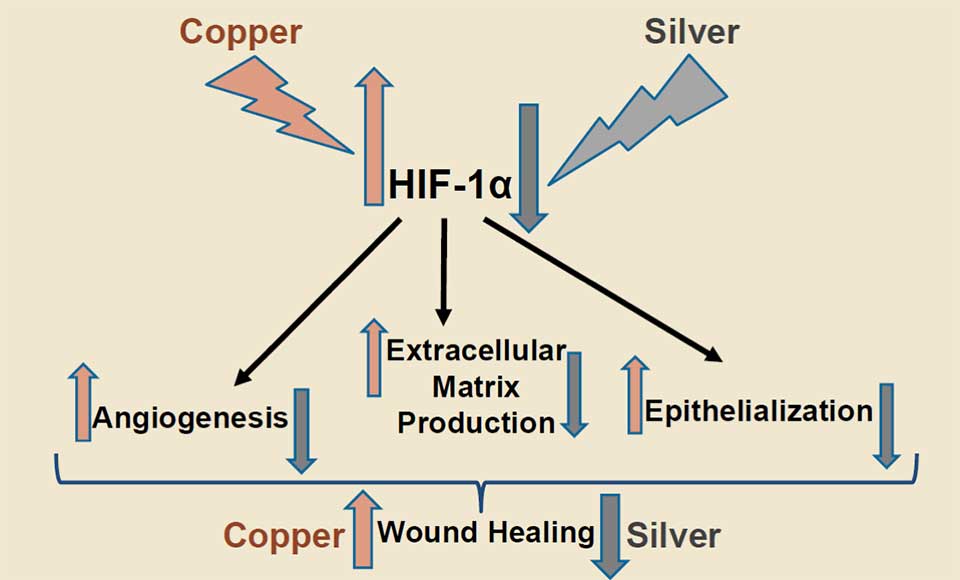
Figure 1. Copper upregulates HIF-1α (6;23;24). The upregulation of HIF-1α induces generation of new blood capillaries (angiogenesis), extracellular matrix production and epithelialization, leading to improved wound healing (25). Silver, in contrast, inhibits the upregulation of HIF-1α (21), impeding wound healing.
The capacity of copper-oxide containing wound dressings to enhance wound closure in non-infected wounds, in contrast to silver wound dressings, is depicted in Figure 2.
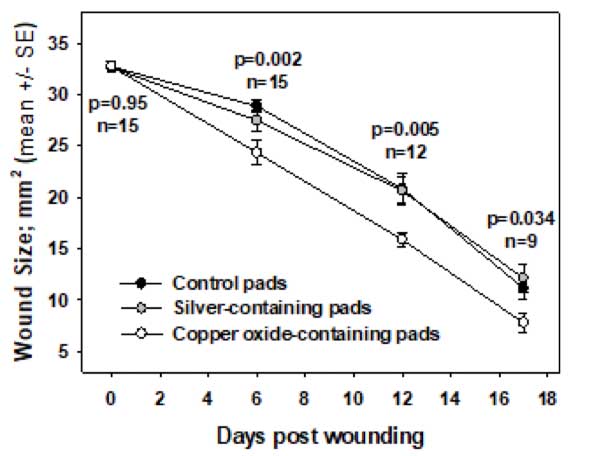
Figure 2. Following circular full-thickness single skin wounds surgically inflicted in the dorsum of diabetic mice, the wounds were directly and continuously covered with control or copper oxide-containing wound dressings or silver-containing wound dressings. The mean wound area ± standard error (SE) at several time points following wounding, the number of the mice examined, and the p values (t-test) between the mean wound areas in the control dressing or copper dressing-treated groups, are depicted (6).
The enhanced wound healing of chronic and hard to heal wounds infected and non-infected wounds, including in wounds that responded poorly to management with silver wound dressings (26), of diabetic patients and other patients, was also demonstrated in the clinic (27-32).
The following Table briefly compares copper and silver in terms of its effect on wound healing and skin related effect.
| Feature / Type | Silver | Copper |
| Wound Healing in General | No direct role in wound healing or any other body physiological function | Essential mineral involved in many of the physiological processes of wound healing |
| Production of Extracellular Matrix (ECM) Skin Proteins | None | Copper stimulates production by dermal fibroblasts of collagen, elastin and other ECM skin proteins |
| Production of blood capillaries (angiogenesis) | None | Copper is involved in the production of growth factors needed for formation of blood capillaries |
| Stabilization of Extracellular Matrix (ECM) Skin Proteins | No | Copper stabilizes the ECM once formed |
| Reduction of Inflammation | No | Copper has anti-inflammatory properties |
| Essential element for the human body | No | Yes. Copper is one of the 11 essential elements the body needs |
| Does the body need these elements to survive | No | Yes |
| Adverse effects due to skin contact | Rare | Extremely rare |
| Toxicity | Rare | Extremely rare |
| Effect of intact skin | Potential argyria (black coloration of the skin) | Improves skin elasticity and improves healthy skin appearance |
| Does the element play a role in our daily diet | No | Official Recommended Dietary Allowance is 0.9 mg/day in the USA |
Antimicrobial activity
As depicted in Figures 3 and 4, and as published (32, 33), copper oxide impregnated wound dressings demonstrated a significant superior antimicrobial efficacy in comparison to most tested silver containing wound dressings against gram positive, gram negative, antibiotic resistant bacteria and fungi.
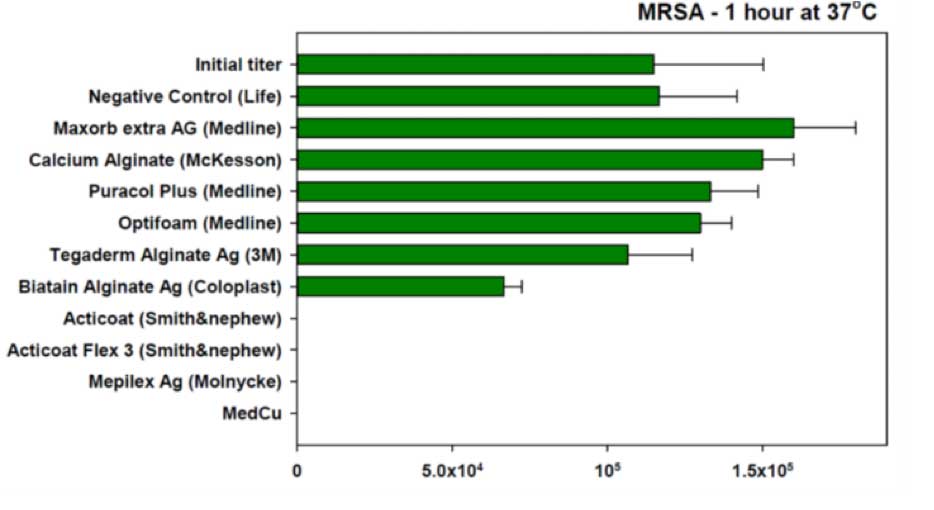
Figure 3. The antimicrobial efficacy of silver wound dressings and copper oxide impregnated wound dressing against Methicillin Resistant Staphylococcus aureus) MRSA( was tested according to the AATCC Test Method 100.
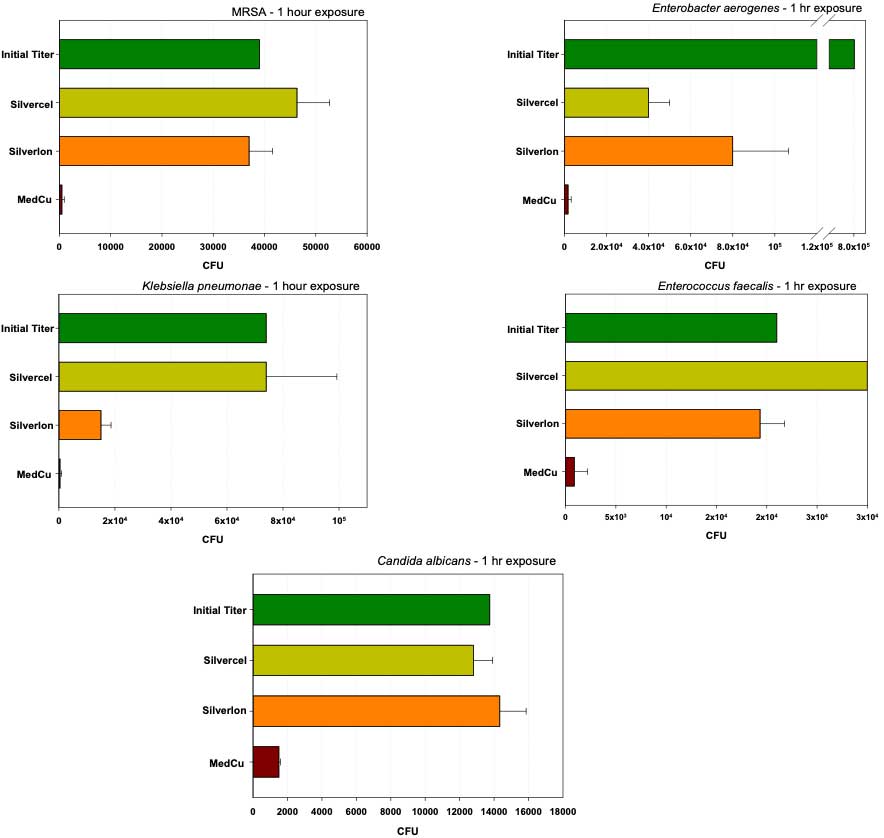
Figure 4. The antimicrobial efficacy of two additional commercially available silver wound dressings and copper oxide impregnated wound dressing was tested according to the AATCC Test Method 100. MedCu wound dressings reduced to a greater degree the initial amounts (titers) of the microorganisms added to the dressings as compared to the tested silver dressings.
In conclusion, copper oxide-containing wound dressing not only confer protection to the wound and the dressing from microbial contamination, and silver dressings do to a lesser degree, but very importantly the copper dressings stimulate skin regeneration and wound healing. This is achieved via the constant release in situ of ppm of copper ions, which in the wound itself stimulate angiogenesis and formation of intense granulation tissue. Thus, copper wound dressings can be used not only on infected wounds (as silver dressings do for a limited time), but can be used in non-infected wounds for very prolonged periods of time until wound closure.
References
(1) Borkow G, Gabbay J. Copper as a biocidal tool. Curr Med Chem 2005; 12(18): 2163-2175.
(2) Uauy R, et al. Essentiality of copper in humans. Am J Clin Nutr 1998; 67(5 Suppl): 952S-959S.
(3) Borkow G, et al. Could chronic wounds not heal due to too low local copper levels? Med Hypotheses 2008; 70(3): 610-613.
(4) Kornblatt AP, et al. The neglected role of copper ions in wound healing. J Inorg Biochem 2016; 161: 1-8.
(5) Parke A, et al. Characterization and quantification of copper sulfate-induced vascularization of the rabbit cornea. Am J Pathol 1988; 130(1): 173-178.
(6) Borkow G, et al. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen 2010; 18(2): 266-275.
(7) Sen CK, et al. Copper-induced vascular endothelial growth factor expression and wound healing. Am J Physiol Heart Circ Physiol 2002; 282(5): H1821-H1827.
(8) Das A, et al. Endothelial Antioxidant-1: a Key Mediator of Copper-dependent Wound Healing in vivo. Sci Rep 2016; 6: 33783.
(9) Tenaud I, et al. In vitro modulation of keratinocyte wound healing integrins by zinc, copper and manganese. Br J Dermatol 1999; 140(1): 26-34.
(10) Harris ED, et al. Copper and the synthesis of elastin and collagen. Ciba Found Symp 1980; 79: 163-182.
(11) Philips N, et al. Beneficial regulation of fibrillar collagens, heat shock protein-47, elastin fiber components, transforming growth factor-beta1, vascular endothelial growth factor and oxidative stress effects by copper in dermal fibroblasts. Connect Tissue Res 2012; 53(5): 373-378.
(12) Ahmed Z, et al. Stabilization of fibronectin mats with micromolar concentrations of copper. Biomaterials 1999; 20(3): 201-209.
(13) Ahmed Z, et al. Stabilisation of cables of fibronectin with micromolar concentrations of copper: in vitro cell substrate properties. Biomaterials 2004; 25(5): 803-812.
(14) Lansdown AB. Metallothioneins: potential therapeutic aids for wound healing in the skin. Wound Repair Regen 2002; 10(3): 130-132.
(15) Rucker RB, et al. Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am J Clin Nutr 1998; 67(5 Suppl): 996S-1002S.
(16) Simeon A, et al. Expression of glycosaminoglycans and small proteoglycans in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu(2+). J Invest Dermatol 2000; 115(6): 962-968.
(17) Simeon A, et al. The tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures. Life Sci 2000; 67(18): 2257-2265.
(18) Chen M, et al. Copper promotes migration of adipose-derived stem cells by enhancing vimentin-Ser39 phosphorylation. Exp Cell Res 2020; 388(2): 111859.
(19) Alizadeh S, et al. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem Biophys Res Commun 2019; 517(4): 684-690.
(20) Borkow G, et al. Copper oxide impregnated wound dressings: biocidal and safety studies. Wounds 2010; 22: 310-316.
(21) Yang T, et al. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: insight into the cytotoxicity and antiangiogenesis. Int J Nanomedicine 2016; 11: 6679-6692.
(22) Khansa I, et al. Silver in Wound Care-Friend or Foe?: A Comprehensive Review. Plast Reconstr Surg Glob Open 2019; 7(8): e2390.
(23) Martin F, et al. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood 2005; 105(12): 4613-4619.
(24) Wu Z, et al. Copper affects the binding of HIF-1alpha to the critical motifs of its target genes. Metallomics 2019; 11(2): 429-438.
(25) Ruthenborg RJ, et al. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol Cells 2014; 37(9): 637-643.
(26) Gorel, O., Hamuda, M., Feldman, I., Kucyn-Gabovich, I. (2024) Enhanced healing of wounds that responded poorly to silver dressing by copper wound dressings: prospective single arm treatment study. Health Science Reports 14;7(1):e1816.
(27) Melamed, E., Kiambi, P., Okoth, D., Honigber, I., Tamir, E., Borkow, G. (2021) Healing of Chronic Wounds by Copper Oxide-Impregnated Wound Dressings – Case Series. Medicina 57, 296. https://doi.org/10.3390/
(28) Melamed E., Rovitsky, A., Roth, T., Assa, L., Borkow, G. (2021) Stimulation of Healing of Non-Infected Stagnated Diabetic Wounds by Copper Oxide-Impregnated Wound Dressings. Medicina 57(10):1129. doi: 10.3390/medicina57101129.
(29) Weitman, C.C., Roth, T., Borkow, G. (2022) Copper to the wound rescue after everything else failed: Case Report. Archives of Clinical and Medical Case Reports 6 (3): 459-466.
(30) Melamed, E., Rovitsky, A., Roth, T., Borkow, G. (2022) Anterior Ankle Full Thickness Skin Necrosis Treated with Copper Oxide Dressings without Debridement and Skin Graft – A Case Report. Archives of Clinical and Medical Case Reports 6: 501-510.
(31) Melamed E., Borkow G. (2023) Continuum of care in hard-to-heal wounds by copper dressings: a case series. J Wound Care 32(12):788-796. doi: 10.12968/jowc.2023.32.12.788.
(32) Borkow, G., Melamed, E. (2021) Copper, an abandoned player returning to the wound healing battle. In: Recent Advances in Wound Healing. Ed: Shahin Aghaei; IntechOpen London: 5 Princes Gate Court, London, SW7 2QJ, UK. Chapter 9, pp. 165-184. ISBN: 978-1-83968-572-9
(33) Borkow, G., Roth, T., Kalinkovich, A. (2022) Wide Spectrum Potent Antimicrobial Efficacy of Wound Dressings Impregnated with Cuprous Oxide Microparticles. Microbiol. Res. 13(3), 366-376. https://doi.org/10.3390/microbiolres13030029